Hello friends,
I hope you had a wonderful week.
This week I was flooded with questions regarding the Pfizer COVID vaccine approval in the United States. I will answer several of your most pressing questions. As always, I strive to provide you with up-to-date and evidence-based information about the pandemic. I appreciate you sharing with friends and family to educate more people with facts, not fiction.
Let’s dive into the bad news, the good news, your questions, and my silver lining for the week.
Dr. Dina, what is the bad news?
There have been almost 250 MILLION cases of COVID-19 worldwide, with more than 5 MILLION reported deaths.
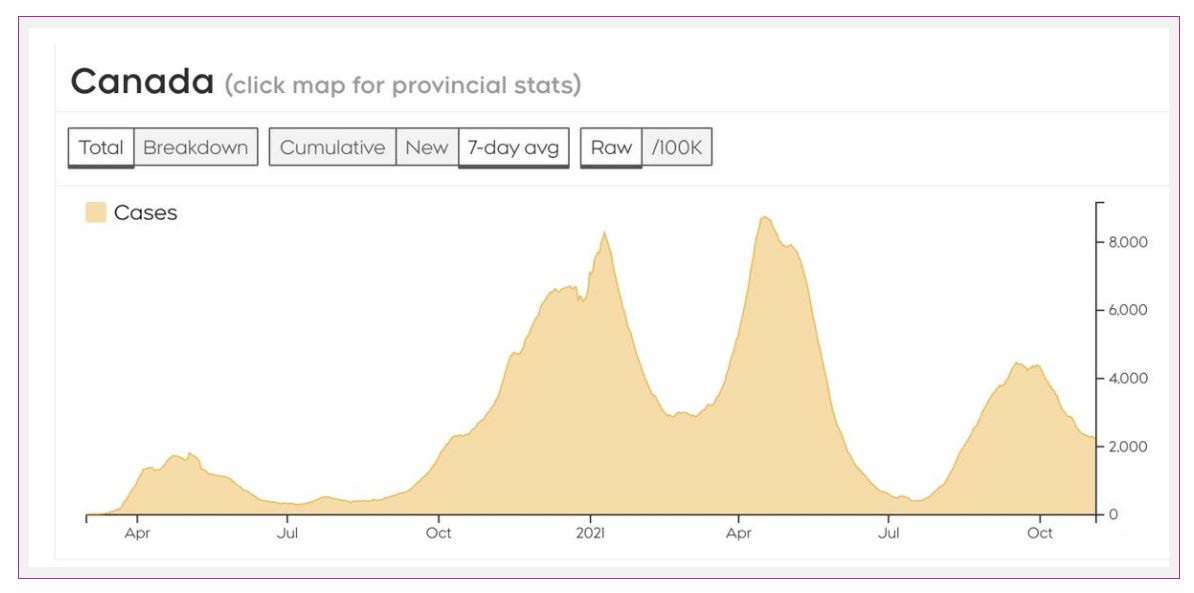
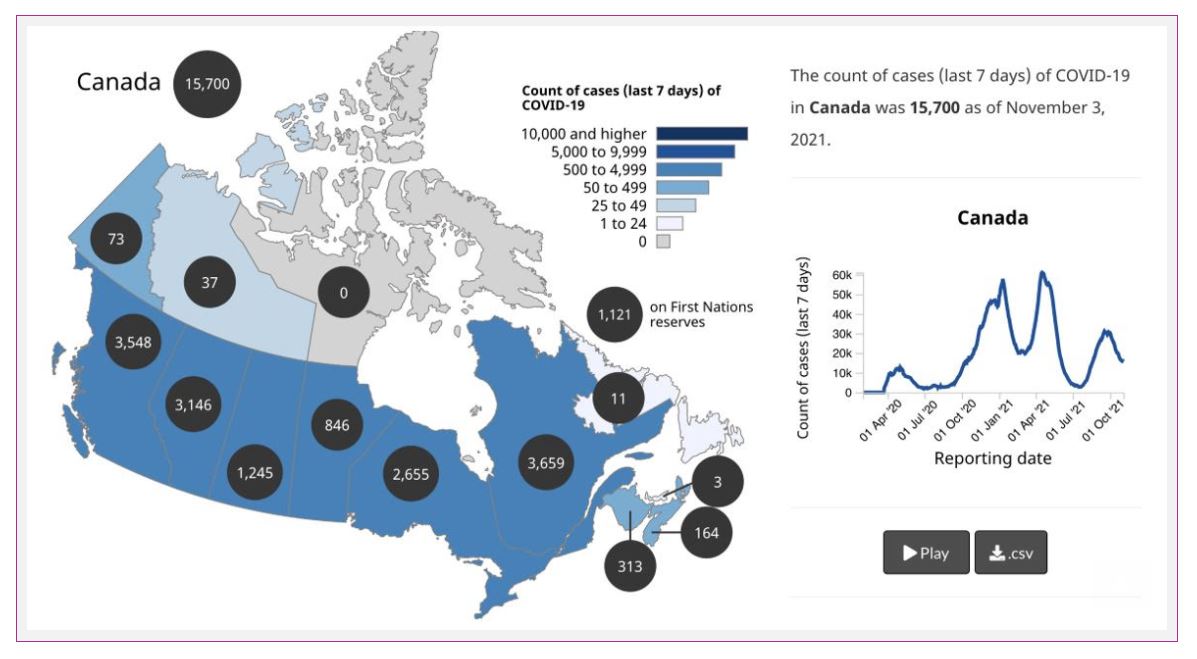
We have diagnosed over 1.7 million cases in Canada, and over 29,000 people have died from COVID-19.
Cases in Canada are pretty stable, though some areas, namely British Columbia, Alberta, Saskatchewan, and Manitoba, are seeing ongoing surges of cases. In addition, cases in the Yukon and Nova Scotia ticked up a bit in the last week.
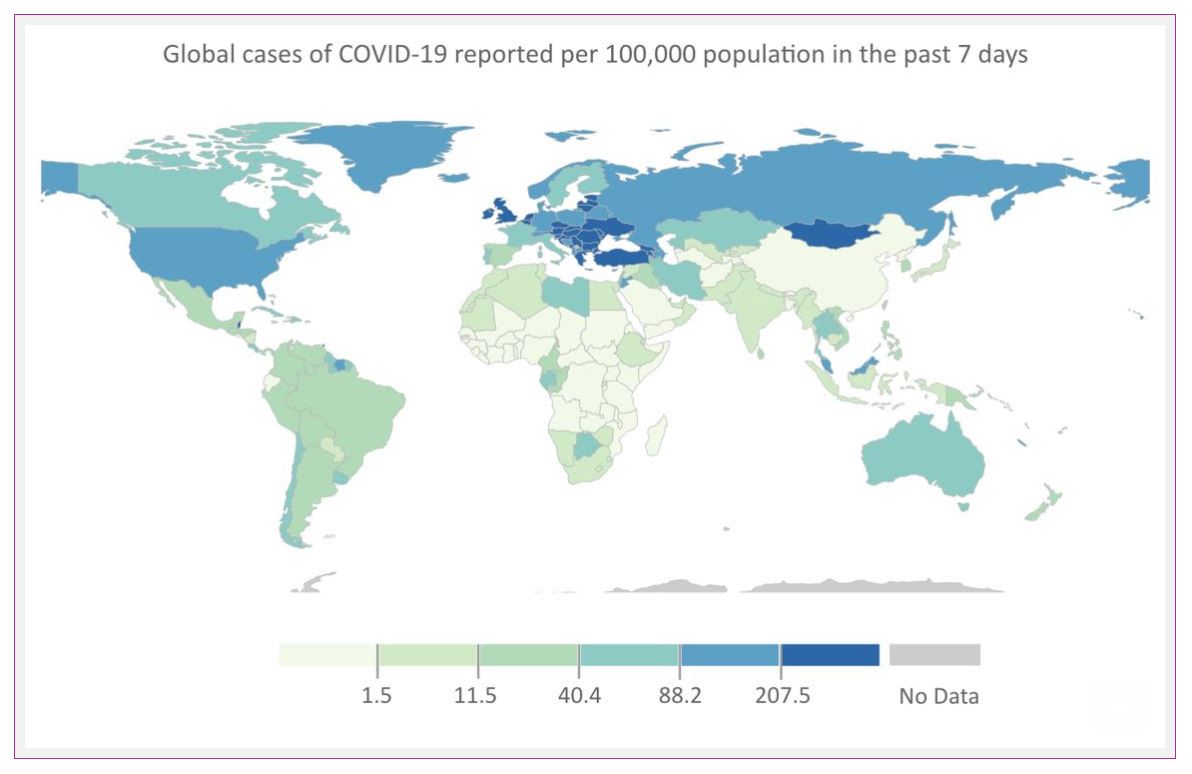
The pandemic seems far from settled in Europe. The World Health Organization expressed ‘grave concern’ last week over the new surge of cases across Europe and Central Asia. Hospitalization rates doubled in the last week.
What is the good news?
Over 7 BILLION COVID-19 vaccines have been given worldwide, representing almost 50% of people receiving their first shot and nearly 40% receiving both. In the whole WORLD. This still blows me away.
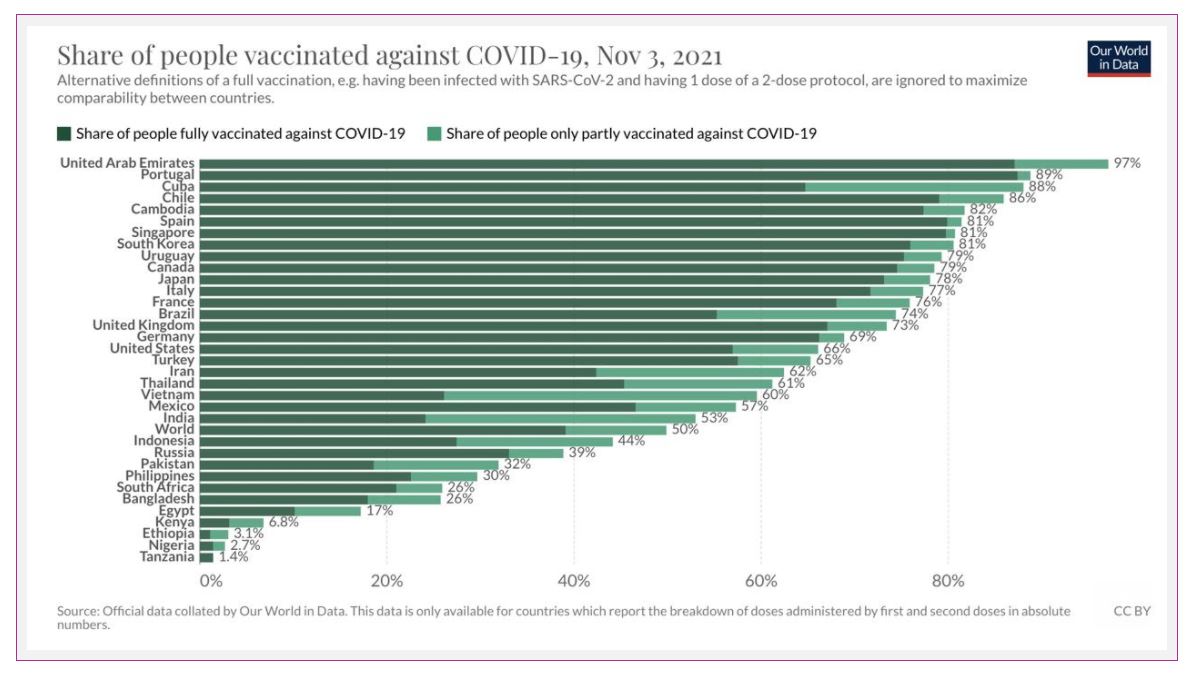
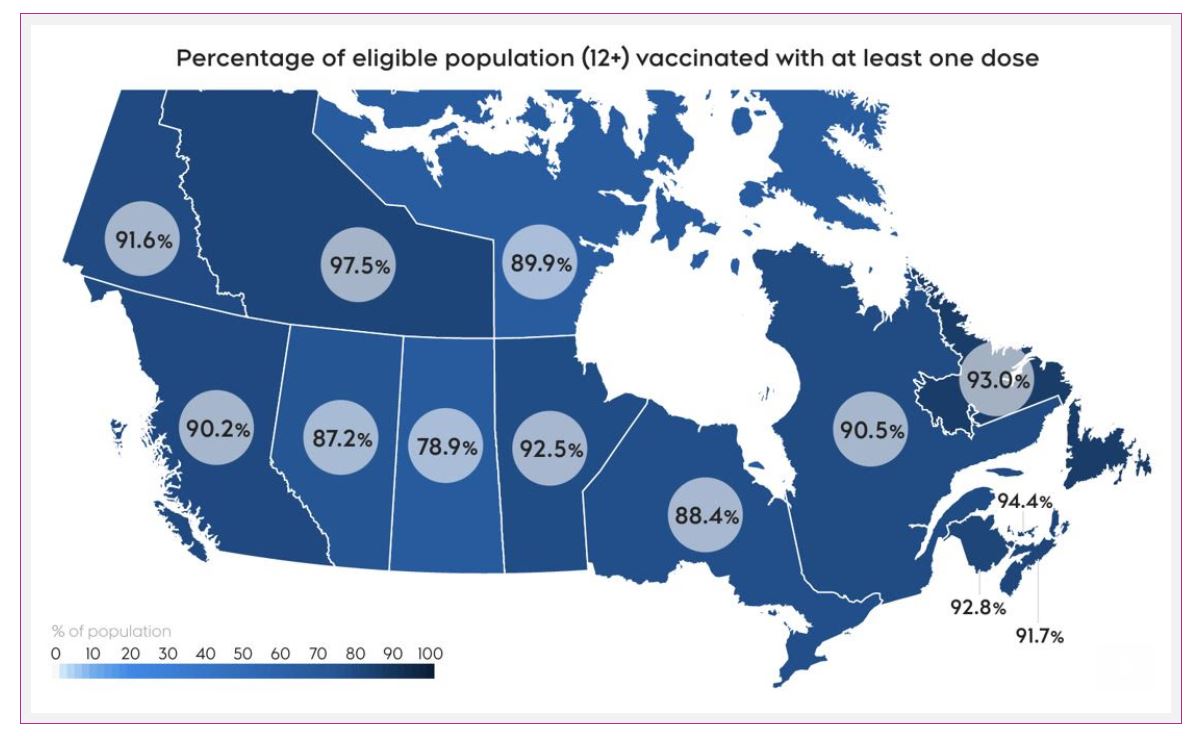
In Canada, 89% of eligible people received one dose, and 85% are fully vaccinated.
This week the U.S.-Canada borders will open for the first time since March 20 to non-essential fully vaccinated Canadians. A negative COVID test will not be required.
The United Kingdom granted conditional authorization for the Merck antiviral pill, molnupiravir. The drug was licensed for adults 18 years of age and older who test positive for COVID and have at least one risk factor for severe COVID-related illness such as heart disease or obesity. Merck announced in September that the medicine decreased hospitalizations and death from COVID by 50%. However, the results have not yet been peer-reviewed or published in a scientific journal.
What’s on everybody’s mind?
NOTE: When I talk about the COVID vaccine for kids, I invariably get hate emails. This section is based on news, evidence; it is objective. We are waiting for Health Canada to approve before this is even an option. If you don’t want to hear about vaccines for kids, please unsubscribe and leave your comments to yourself. Thanks.
The US Centers for Disease Control and Prevention recommended the Pfizer COVID-19 vaccine for kids aged 5-11. The U.S. FDA approved its emergency use the week prior.
Some children in the U.S. have already received the vaccine.
Your most common questions of the week
What dose of the Pfizer COVID-19 vaccine did the FDA and CDC authorize for children ages 5 to 11?
The dosage authorized for this age group is ten micrograms. This is one-third the dose given to adults and kids 12 and older.
How long will it take for children to be considered fully vaccinated?
Two vaccine doses will be given, spaced 21 days apart or longer, like teens and adults. Children are considered fully vaccinated two weeks after receiving the second dose.
What side effects are expected?
In the clinical trial of over 3000 kids who received the vaccine, the side effects were similar to those experienced by teens and adults. The most common side effects are fatigue, headache, and soreness at the injection site. In addition, some kids can experience fever and chills. Side effects subsided after a few days.
Importantly, these side effects were less common and less severe in younger kids than adults, likely due to the lower dose.
Anecdotally – I have asked many of my teenage patients if they experienced side effects when they received their shots. Almost none had any side effects at all. They are either far tougher than other adults and me, or kids don’t suffer the same side effects as adults. Those robust immune systems seem to protect them.
In the clinical trial, there were no cases of pericarditis or myocarditis. This side effect has been seen more commonly in teens and young adults and is very rare. The risk of myocarditis from COVID-19 is expected to be higher than the risk of myocarditis secondary to the vaccine.
Some parents tell me they are worried about long-term vaccine side effects. However, there is no scientific reason to think these will occur. Immunization-related side effects typically occur within the first 1-3 weeks following vaccines, not months afterward.
Can a child get the COVID vaccine simultaneously with another vaccine, such as the flu vaccine?
Yes. The COVID vaccine can be given with the flu shot or any other vaccine. If offered at the same time, each vaccine would be delivered to a different injection site (such as one in each shoulder or thigh). The COVID vaccine can be given before or after other vaccines, and there is no need to delay administration based on previous or upcoming vaccines.
Should a child with a history of food or medication allergies avoid the COVID vaccine?
Food or medication allergy is not a contraindication to COVID vaccination. The only reason not to get the COVID vaccine is in the case of a severe allergic reaction to one of this vaccine’s components.
COVID vaccines do not contain egg products.
If your child has severe allergies, please review with your allergist.
Should a child who has had COVID before still get vaccinated?
Health Canada recommends that adults and teens with a previous infection get the vaccine. Regarding kids’ vaccines, the CDC recommends that kids receive two doses of the vaccine, regardless of prior infection. Vaccination provides additional, longer-lasting protection than antibodies from the illness itself.
Should 11-year-olds wait until they turn 12 to get the higher dose?
This was reviewed by the CDC extensively. The CDC recommends that children receive the appropriate dose for their age at the time of vaccination. For example, if a child is about to turn 12, they could get the ‘kids 10-microgram dose’ for the first shot and the ‘adult 30-microgram dose’ for the second.
In studies, the 10-microgram dose was the optimal balance in effectiveness and lesser side effects. Thus, there likely is no significant difference between the ‘kids’ versus the ‘adult’ dose for 11 to 12-year-old children.
My silver lining of the week
This week I want to highlight my incredible family at Kidcrew. The last two years have been challenging for all of us, and I am so proud that I am supported by the most dedicated, professional, efficient, and compassionate #TeamKidcrew.
Within two weeks of receiving our first shipments of the flu shot, every single one has been given, and thousands of kids are protected. The team worked 7 days a week, from early in the morning to late in the evening, to make that happen. I am grateful for my colleagues every day.
If your child is a current primary care patient at Kidcrew, you can book your flu vaccine appointment online. Go to the Book Online tab to book your child’s appointment. Please register in one spot only, and ensure you can bring your child at this time. We have many families looking to book an appointment and are awaiting more flu vaccine doses. If you schedule an appointment, please be sure you can attend to avoid a missed opportunity for another family. If you do not see any open appointments, please check back as we will be opening more spots as we receive more flu vaccines.
Have a wonderful week, friends!






![[Dr. Dina News] IMPORTANT UPDATE re. VIRTUAL CARE](https://drdina.ca/wp-content/uploads/2021/01/dr-dina-kulik-kids-and-virtual-care-1a-400x250.jpg)
![[Dr. Dina News] COVID-19 Vaccine for Infants and Young Children.](https://drdina.ca/wp-content/uploads/2021/04/dr-dina-kulik-kids-and-vaccines-400x250.jpg)
![[Dr. Dina News] COVID-19 Vaccine for Infants and Young Children.](https://drdina.ca/wp-content/uploads/2022/04/DRD-1-400x250.jpg)






