Hello friends!
I hope you had a wonderful, shorter work week and a great weekend.
Let’s dive into the bad news, good news, most common questions of the week and end on a sweet silver lining.
What is the bad news?
There have been over 430 MILLION cases of COVID reported worldwide, with almost 6 million deaths attributable to COVID-19.
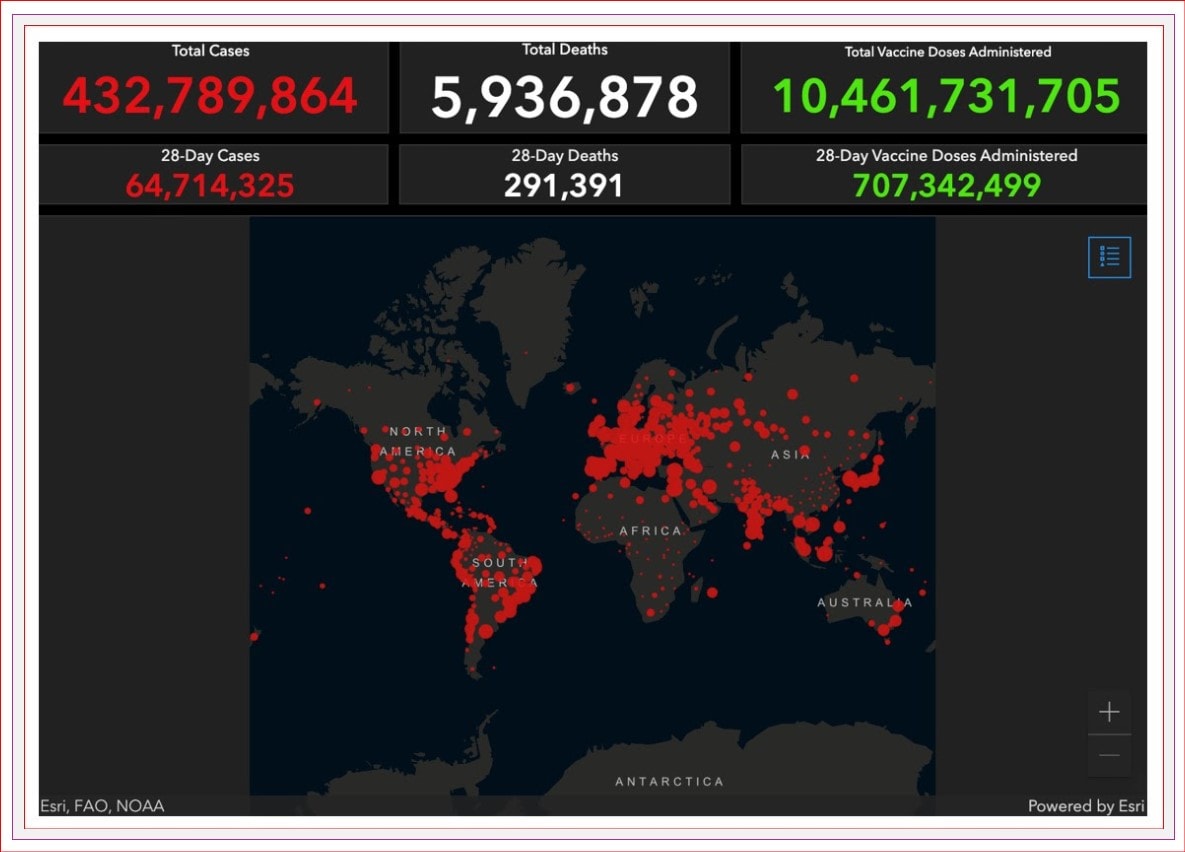
We have almost 3.3 million reported cases in Canada, with over 36,000 deaths. There are over 114,000 active COVID cases in Canada, with over 5,500 hospitalizations.
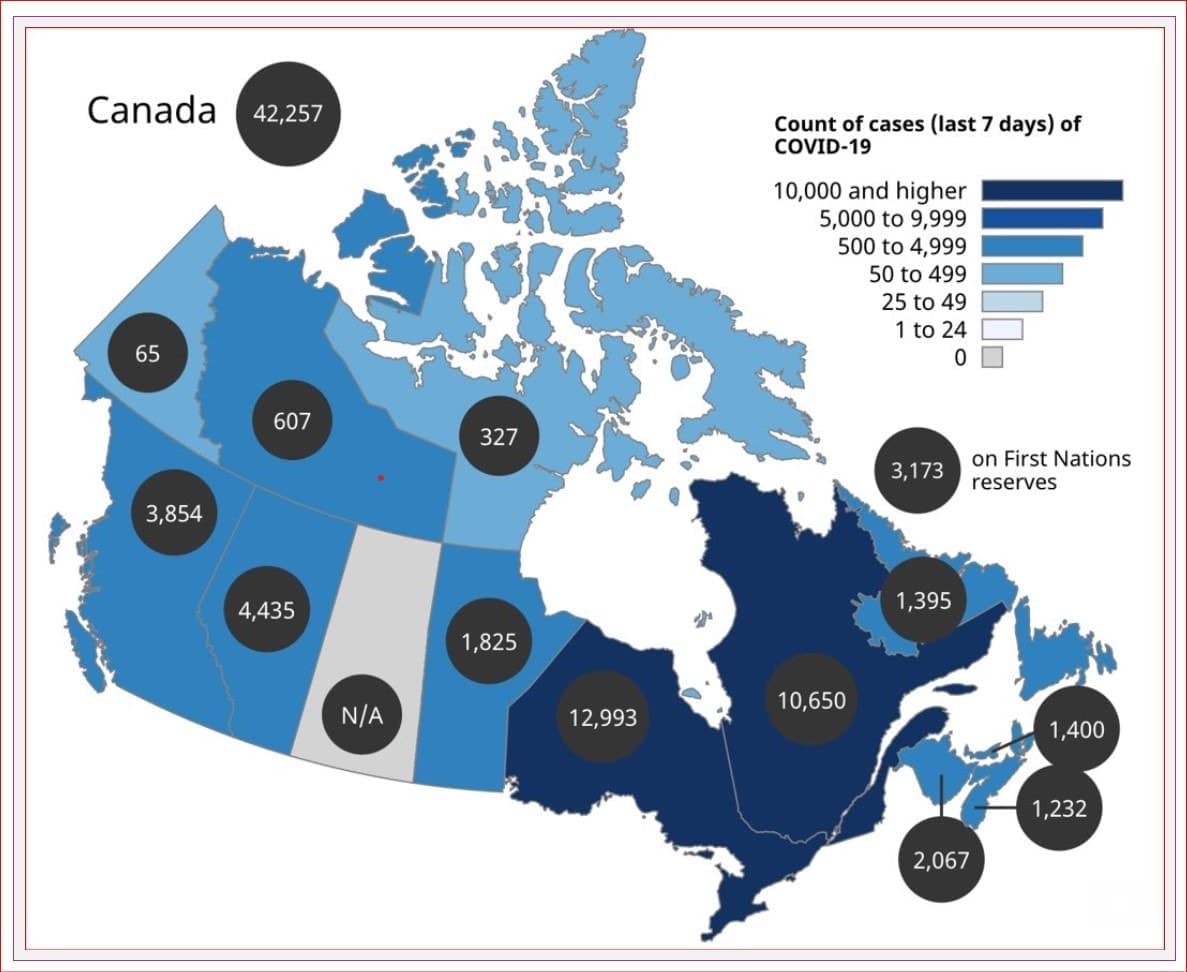
What is the good news?
Reported cases, hospitalizations and deaths are declining across Canada. Many provinces are moving forward with their plans to reopen and decrease restrictions.
COVID is still infecting many people, including Queen Elizabeth II, Prince Charles and his wife Camilla, last week. The United Kingdom removed all COVID-19 restrictions last week. People with active COVID infections no longer have to self-isolate.
Australia opened their borders to international tourists provided they are vaccinated.
The situation is not as favourable in Hong Kong, where they are experiencing the worst COVID wave yet, with over 5000 new infections a day. Their health care system is at risk of collapse.
Worldwide, over 10.6 BILLION vaccines have been given, representing 63% of the world’s population receiving their first dose and 55% receiving 2.
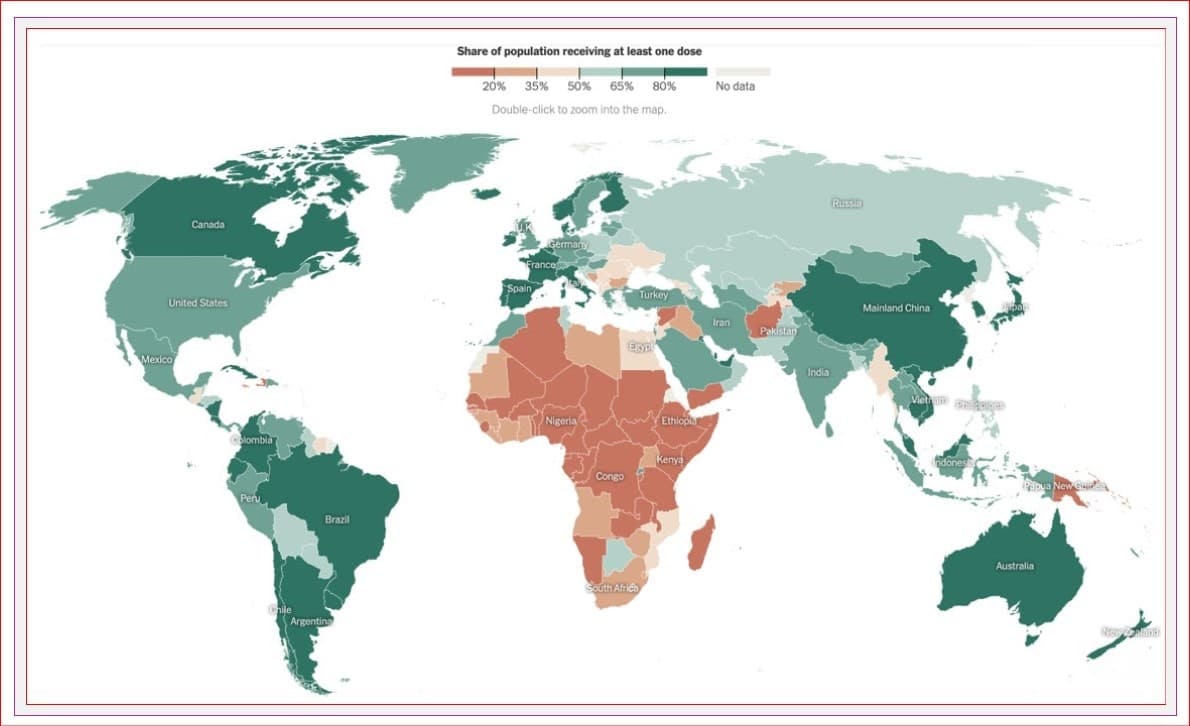
In Canada, 89% of people five and older have received their first dose of a COVID vaccine, and 85% have received at least two.
The most common questions of the week
Is Omicron less likely to cause severe illness?
While we have seen a significant wave of Omicron cases over the last few months, this did not lead to as many severe cases or deaths as was feared.
A recent study from the Korea Disease Control and Prevention Agency (KDCA) looked at 67,200 infections since December 2021. It showed that people infected with Omicron are 75% less likely to develop severe illness requiring ICU hospitalization or death than those infected with the Delta strain.
What is Novavax Nuxaxovid?
The Novavax Nuvaxovid COVID-19 vaccine was approved for use by Health Canada on February 17.
Nuvaxovid is a recombinant protein subunit vaccine and not mRNA based. This is a new option for those unable or not willing to receive an mRNA vaccine.
The National Advisory Committee on Immunization (NACI) continues to preferentially recommend mRNA vaccines over other types of COVID vaccines due to known safety and efficacy profiles. Nuvaxovid provided 90.4% protection against COVID infection and 100% protection against moderate and severe disease in clinical trials. This data does not include evidence of effectiveness against Delta or Omicron variants, as the trials were conducted before these variants emerged.
This new vaccine is expected to arrive in Ontario in mid-March.
What is Evusheld?
Evusheld is a pre-exposure preventative antibody treatment for COVID, made by AstraZeneca. This therapy is under review by Health Canada, and the Canadian government has signed an agreement to procure 100,000 doses of it.
If approved, it will be used as an antibody therapy for special populations, such as those who are immunocompromised. In the United States, it is authorized for use by people with moderate-severe immunodeficiency or those with a history of severe adverse reactions to the COVID vaccine. It is to be used in those who are not currently infected.
Evusheld is given as two intramuscular injections (like the COVID vaccines) and can help prevent illness for six months.
What is Covifenz?
Covifenz is the new vaccine on the block. It is developed by Medicago, a biotech company in Quebec City. This vaccine uses a plant host to make spike-like proteins that trick the body to create antibodies against COVID-19.
While many other vaccines use virus-like particles, this vaccine is the first to use plant-based technology.
The vaccine series will consist of 2 shots, administered three weeks apart. The vaccines were 71% effective at preventing COVID infection one week after the second dose but 100% effective at preventing severe disease. The study involved 30,000 participants.
Covifenz can be used in people aged 18-64. We do not know if it is safe or effective in people younger than 18 or older than 64.
Side effects were similar in those that received the vaccine and those who received a placebo.
The vaccine is expected to be available by May 2022.
The National Advisory Committee on Immunizations (NACI) is expected to provide guidance on using this vaccine in the coming weeks.
Did CDC catch up to NACI?
A few weeks ago, I let you know that NACI advised an eight-week interval between the first and second doses of COVID-19 mRNA vaccines. The CDC announced last week that they, too, recommend this dosing schedule.
An extended time between the first and second doses may reduce the small risk of myocarditis/pericarditis, especially among young men.
The 8-week schedule also allows for higher antibody levels and longer-lasting immunity than a shorter interval between doses.
A three-week interval between the first and second dose of the Pfizer vaccine and 4-week interval for the Moderna vaccine is still recommended by the CDC for immunocompromised people, those older than 65 and others at increased risk of severe disease’.
Is MIS-C less common in vaccinated children?
A recent study published in The Lancet Child & Adolescent Health shows that multisystem inflammatory syndrome is rare in vaccinated kids, just 1 case per million. They found that 21 kids required hospitalization for MIS-C out of 20 million people aged 12-20 who received at least one COVID vaccine.
The silver lining of the week
As cases continue to fall and we no longer feel the pinch of an overburdened healthcare system, many of us are slowly starting to relax. I feel more positive energy amongst parents and kids. Many of you are beginning to socialize more again, attend extracurriculars, playdates, and even travel. I see fewer parents with their shoulders by their ears with the constant threat of illness and uncertainty. While the pandemic is not over, with the relaxation of public health restrictions, and the mildness of Omicron, I can see the spark of joy and calm again on the (masked) faces of my patients and families.
It is wonderful to experience.
Have a wonderful week, everyone!

![[Dr. Dina News] IMPORTANT UPDATE re. VIRTUAL CARE](https://drdina.ca/wp-content/uploads/2021/01/dr-dina-kulik-kids-and-virtual-care-1a-400x250.jpg)
![[Dr. Dina News] COVID-19 Vaccine for Infants and Young Children.](https://drdina.ca/wp-content/uploads/2021/04/dr-dina-kulik-kids-and-vaccines-400x250.jpg)
![[Dr. Dina News] COVID-19 Vaccine for Infants and Young Children.](https://drdina.ca/wp-content/uploads/2022/04/DRD-1-400x250.jpg)






